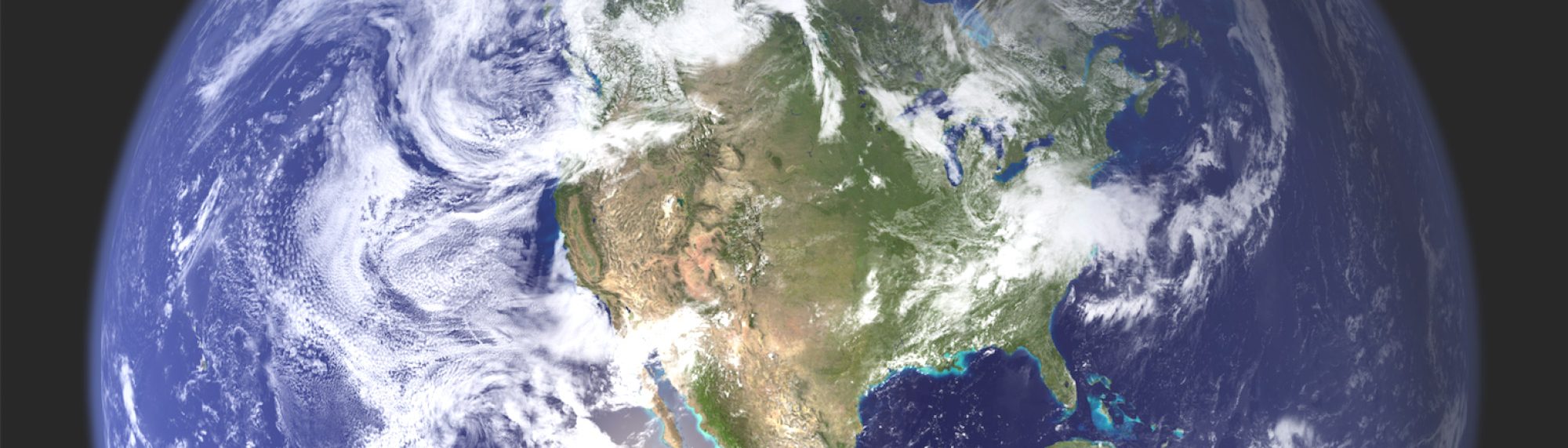
January 5, 2002 New York City, New York - In a Dreamland radio news report at the end of 2001, I talked with a Columbia University scientist about the risk of rapid global climate change as cars and industries put more and more carbon dioxide and other greenhouse gases into the atmosphere. (See Earthfiles 12/22/01.) Industries and motor vehicles over the last century between 1900 and 2000 put 280 billion tons of carbon from carbon dioxide into the earth's atmosphere and oceans. That blanket of CO2 is warming the planet now and is expected to keep getting thicker and warmer throughout the next hundred years. The unpredictable consequence could be rapid global climate change and many plant and animal extinctions.
Click here to subscribe and get instant access to read this report.
Click here to check your existing subscription status.
Existing members, login below:
© 1998 - 2024 by Linda Moulton Howe.
All Rights Reserved.