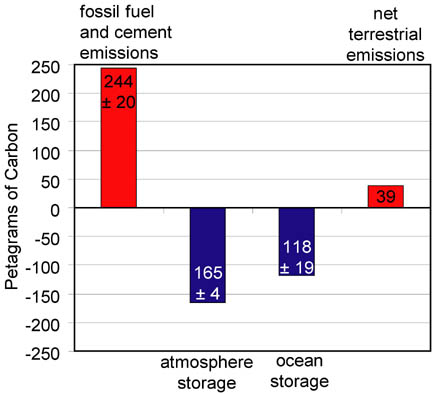
August 13, 2004 – The National Oceanic and Atmospheric Administration (NOAA) recently released the results of a 15-year-long study to measure the total amount of dissolved carbon in the oceans specifically carbon from rapidly increasing carbon dioxide emissions produced by human industrial activities. The startling finding is that between the year 1800 the beginning of the Industrial Age and the year 1994, the world’s oceans absorbed 118 billion metric tons of carbon! That is roughly HALF of all the CO2 that has been released from burning fossil fuels and cement manufacturing during those 194 years, according to Professor Christopher Sabine, Oceanographer at the University of Washington in Seattle. He is also on the oceanography staff of NOAA’s Pacific Marine Environmental Laboratory, where he has worked for five years since beginning the ocean water sampling studies at Princeton University back in 1992. I talked this week with Dr. Sabine about the amount of carbon absorption he and his colleagues found and what the impact could be on ocean chemistry and sea life.
Interview:
Christopher Sabine, Ph.D., Professor of Oceanography, University of Washington, and Oceanographer, NOAA’s Pacific Marine Environmental Laboratory, Seattle, Washington: “What we found is that between 1800 and 1994, the oceans have taken up 118 billion metric tons of carbon which is roughly 48% of the CO2 that has been released from burning fossil fuels and cement manufacturing.
Most people don’t appreciate the fact that the oceans today are taking up about 20 million metric tons of carbon every day, just from the fact that we are releasing it into the oceans. That is all entering the oceans through the surface of the oceans. The surface layers of the oceans are where most of the life in the oceans occurs. That’s where the plants in the oceans grow and where the organisms are that feed on them. So we find much more life abundant in the surface layers, which are being the most strongly affected by the CO2 coming in from the atmosphere.
This work is kind an culmination of fifteen years worth of work. We spent ten years doing a very intensive survey of the global oceans, making 72,000 samples of seawater from 10,000 different places. Then it took five years to pull all these data together.

IS IT TRUE THAT WE HAVEN’T HAD THESE KINDS OF GLOBAL WARMING CONDITIONS AND THE ISSUE OF OCEANS ABSORBING CARBON DIOXIDE AT THIS RATE FOR AT LEAST 20 MILLION YEARS?
That’s right. And that’s ultimately the problem. We’re moving into an environmental situation that is unprecedented in our experience as humans. Therefore, predictions of what will happen as a consequence of that are very difficult and very uncertain. We have no precedent for what has happened in the past.
HOW SERIOUS COULD THIS GET OVER THE NEXT 20 TO 25 YEARS?
Again, that’s a tough question because the oceans are very dynamic systems. There are millions of different organisms that are all competing with each other and if the CO2 has a stronger impact on one species relative to another, that might change the competitive advantage of that species and might change the distribution. But it’s not clear exactly what consequence that might have for the future.
There are predictions and models, of course, but it’s hard to say what the ultimate consequences will be until we actually experience them, or have a better understanding of the dynamics of these different Eco-systems.
Ocean Carbonic Acid Can Dissolve
Protective Shells of Sea Creatures
COULD YOU EXPLAIN ONE OF THESE PROBLEMS WHICH IS, AS WATER IN THE OCEANS ABSORBS CARBON DIOXIDE THAT IT DOES FORM CARBONIC ACID WHICH DOES HAVE AN IMPACT ON ANY KIND OF SHELL FISH?
CO2 is an acid gas. When the CO2 dissolves into the ocean, it reacts with a water molecule to make carbonic acid which is H2CO3. That then disassociates into a proton and bicarbonate which is HCO3 with a minus one charge.
That then disassociates into what we call “carbonate ion” which is CO3 minus two charges.
Organisms that produce shells such as mollusks, clams and a number of free floating organisms, small plankton organisms that generate shells not only the large clams and mussels on the shore. Also coral reefs produce their shells from calcium carbonate. That’s calcium and carbonate. So, they take a calcium ion out of the water and they take a carbonate ion out of the water and they put it together to make a solid shell. As CO2 enters the ocean, what it does is the CO2 you can think of the carbonate ion as like an “anti-CO2.” When you combine a CO2 and a carbonate, they neutralize each other. So, as we are adding CO2, we are removing the carbonate ion that is currently in the ocean that the organisms in the oceans use to produce their shells.
As we continue to remove that carbonate, it becomes more difficult for those organisms to make their shells, and when we look at those shells under a microscope, we can see they are malformed.
So, these organisms have difficulty producing their shells which might change their competitive advantage in the oceans.
COULDN’T IT MEAN THAT ALL SHELL CREATURES COULD BE EXTINCT BY THE END OF THIS CENTURY?
We don’t understand exactly how quickly they can adapt and that’s where it is significant that not only the CO2 levels we are experiencing have been seen in the past, in the geological history many millions of years ago. But what is significant here now is the rate of change. We do not believe that the CO2 concentrations changed at this modern rate in the past.
MEANING SO QUICKLY.
And that affects how quickly organisms can adapt and how well they can adapt to these changing environmental conditions. That might have serious consequences.
SO, THE FASTER IT CHANGES WHICH IS WHAT IS HAPPENING NOW, THE MORE DIFFICULT IT MIGHT BE FOR THE SHELL FISH CREATURES TO ADAPT AND SURVIVE?
That’s right.
WHAT HAPPENS OVER THE NEXT 100 YEARS IF WE ARE NOT ABLE TO SEQUESTER CARBON DIOXIDE AND THE OCEANS CONTINUE TO TAKE IT UP AND BECOME MORE CARBONIC ACID AND THE SHELLS DISSOLVE ON SHELL FISH AND ALL OF THAT. WHAT IS THE WORST CASE JUST 100 YEARS FROM NOW IF WE DON’T DO SOMETHING?
You know, it’s very difficult to say what really is going to happen. Right now, we are increasing atmospheric CO2 at an exponential rate. It’s increasing faster and faster and as long as the atmospheric CO2 continues to go up, the oceans will continue to absorb more and more CO2 and that will likely have consequences on the organisms. If we add on top of that a warming planet where we are warming the atmosphere and the oceans we are changing the environment and any time we change the environment like that, it will have consequences for the food webs and the structures of organisms. So, there will likely be dramatic changes in the world as we know it if we continue to release CO2.”
Carbon Sequestration Ideas
Currently, Dr. Sabine is preparing a special report for the United Nation’s Intergovernmental Panel on Climate Change to assess the state of human science and technology required for carbon sequestration including getting it transported from industries down deep in the oceans.
Prof. Sabine: “The idea is that if we can instead of releasing the CO2 into the atmosphere if we can capture that CO2 and put it into the deep ocean, then we might have less of an impact on the surface life and that carbon would be stored in the oceans for a longer period f time.
HOW WOULD YOU DO THAT? HOW WOULD YOU STORE IT DEEP?
The ocean, as you go deeper, it gets colder and the pressure increases. There is a point at which the carbon dioxide actually becomes more dense for example, if you were to take liquid carbon dioxide, it would be more dense than sea water. so, if you can get that CO2 down to that level which in the Pacific would be around 2,000 to 3000 meters, which is about 9,000 feet. If you can get the CO2 that deep, then the liquid CO2 would sink rather than rise to the surface.
BUT HOW DO YOU GET IT DOWN THAT FAR?
They talk about building pipelines or having ships that do it. There have been any number of ideas. If you are familiar with dry ice, which is frozen CO2, there are ideas that you could take huge blocks of dry ice and push them into the oceans. Dry ice is more dense that water, so it would sink and eventually it would fall down into the bottom of the ocean before it completely dissolved. That would be another way of sequestering.
The problem is that these are expensive approaches and right now it looks like some of the storage in geological reservoirs are much more feasible and cost effective than storage in the oceans. That means pumping CO2 deep down into the ground under rock layers.”
Pteropod Shells Dissolve in Ocean Water
Affected by High Carbon Absorption
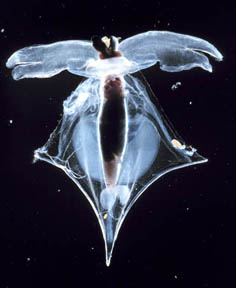
Another scientist has gone to ocean waters where small snail-like animals called Pteropods build their protective shells from aragonite. Aragonite is a form of calcium carbonate that is highly soluble in sea water. The research was done by Prof. Victoria Fabry in the Dept. of Biological Sciences at California State University San Marcos. One of the surprises from her study of the Pteropods was how quickly the shells began to dissolve where carbonic acid has been produced by the high carbon absorption of the ocean water.
Victoria Fabry, Ph.D., Professor of Biological Sciences, Dept. of Biological Sciences, California State University, San Marcos, California: “I looked at one group of calcifying plankton which are the Pteropods. Pteropods are planktonic snails that secrete calcium carbonate shells that are made out of aragonite, which is a form of calcium carbonate that is highly soluble in sea water. What I found was that when these animals were in water that was under-saturated (not enough carbonate ions to make shells) with respect to aragonite, their shells started to dissolve within 48 hours. the animals were still alive and they were actually swimming. But yet, I could see that their normally transparent shells were dissolving. They become white and opaque around the growing edge of the shell when they start to dissolve.
It’s very hard to predict what the ecological consequences are going to be, but it’s likely we might see shifts in the species compositions in certain Eco-systems. Some Pteropod species might have to shift their geographical range. In this case, they might shift closer to the equator toward waters that are super-saturated with respect to aragonite.
WHICH WOULD BE WARMER WATERS.
Yes.
AND THAT MEANS THERE MIGHT BE A WHOLESALE CHANGE OF POPULATIONS THAT COULD PRECIPITATE A COLLAPSE OF SPECIES THAT MIGHT GO TO EXTINCTION.
I hesitate to use the word extinction, but what we might find is that some Pteropods are excluded from areas where waters are under-saturated and it looks like those are going to be near the poles in the sub-Arctic Pacific and the Southern Ocean near Antarctica. It looks like it’s going to happen there the soonest and Pteropods in both of those areas do play an integral part in the food web. But what that ultimate consequence would be, we don’t know. The only thing we can say is that there will be changes.”
More Information:
Role North Atlantic Drift Is Playing
in CO2 Ocean Absorption
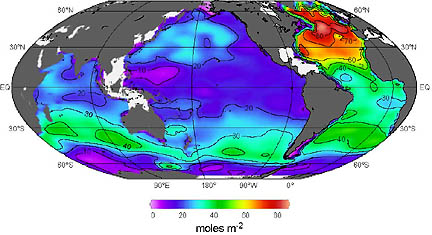
Professor Sabine: “The graphic is what we call a ‘column inventory’ of CO2 in the oceans. So if you take each square meter of the ocean and add up all the manmade CO2 that’s accumulated between the surface and the bottom in that area of the ocean, you can see that there are areas that have very little CO2 and other areas that have quite a lot of CO2. There are large spatial differences, depending upon where we are, and one area that has a tremendous amount of CO2 is in the North Atlantic. The reason for that is it’s an area where water is dragging CO2 down into the deep ocean. So one of the findings of our paper is that half of all the manmade CO2 that has been introduced to the oceans can be found just in the upper 10% of the ocean depth.
The average global ocean is about 3800 meters deep. We found that half of all the CO2 that has been introduced is found in the upper 400 meters. So, most of the deep ocean has NEVER been exposed to the manmade CO2. There are a few exceptions to that and these are in areas where for example in the North Atlantic where you take warm, salty water from the tropical Atlantic that moves north as part of the Gulf stream. It moves north, gets cooled off, becomes more dense and sinks to the bottom. As it moves north, it’s also absorbing that manmade CO2 that we released and when it sinks, it takes that CO2 with it. In the North Atlantic is one area where we have seen manmade CO2 all the way to the bottom at 2 miles depth we’ve seen manmade CO2.
ALL THE WAY DOWN TO 2 MILES. AND YOU ARE DESCRIBING THE NORTH ATLANTIC DRIFT WHICH IS ALSO RESPONSIBLE FOR HELPING TO KEEP EUROPE AND ENGLAND WARMER.
That’s right.
SO, NOT ONLY IS IT HELPING RIGHT NOW WITH THE CLIMATE AS LONG AS THAT NORTH ATLANTIC DRIFT KEEPS MOVING, BUT IT IS TAKING CO2 DOWN AT LEAST TWO MILES.
Right, and that large scale circulation is very important for the oceans taking up CO2. So, if we were to change that in the future, that will have a dramatic impact on how much CO2 the oceans can absorb.”
© 1998 - 2024 by Linda Moulton Howe.
All Rights Reserved.